The Medefil Story
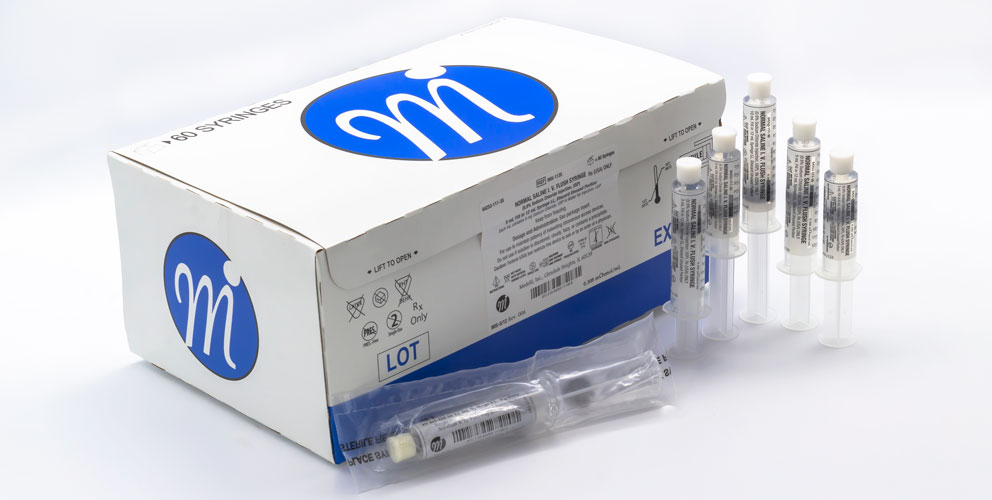
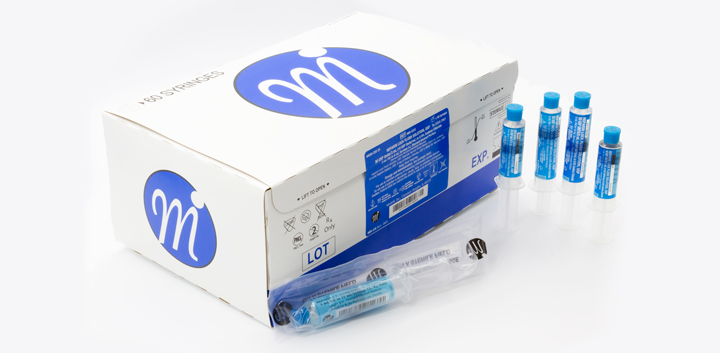
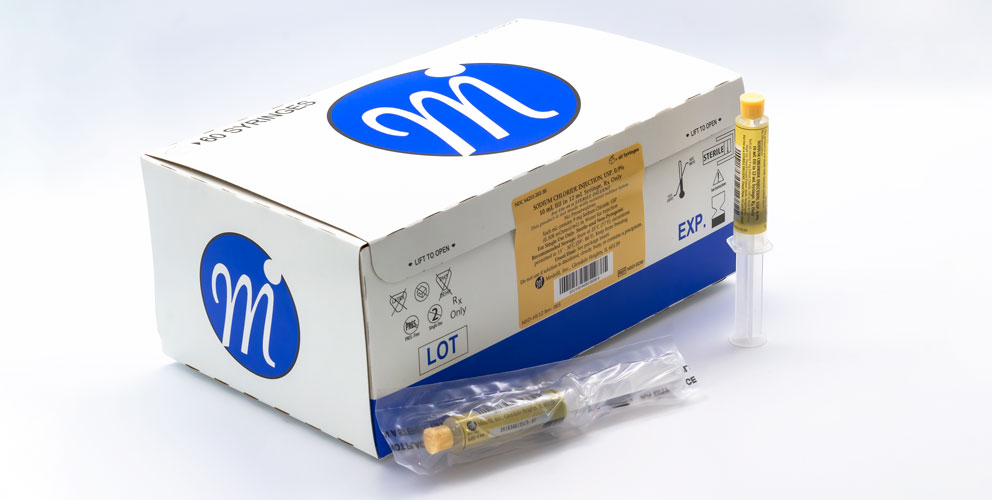
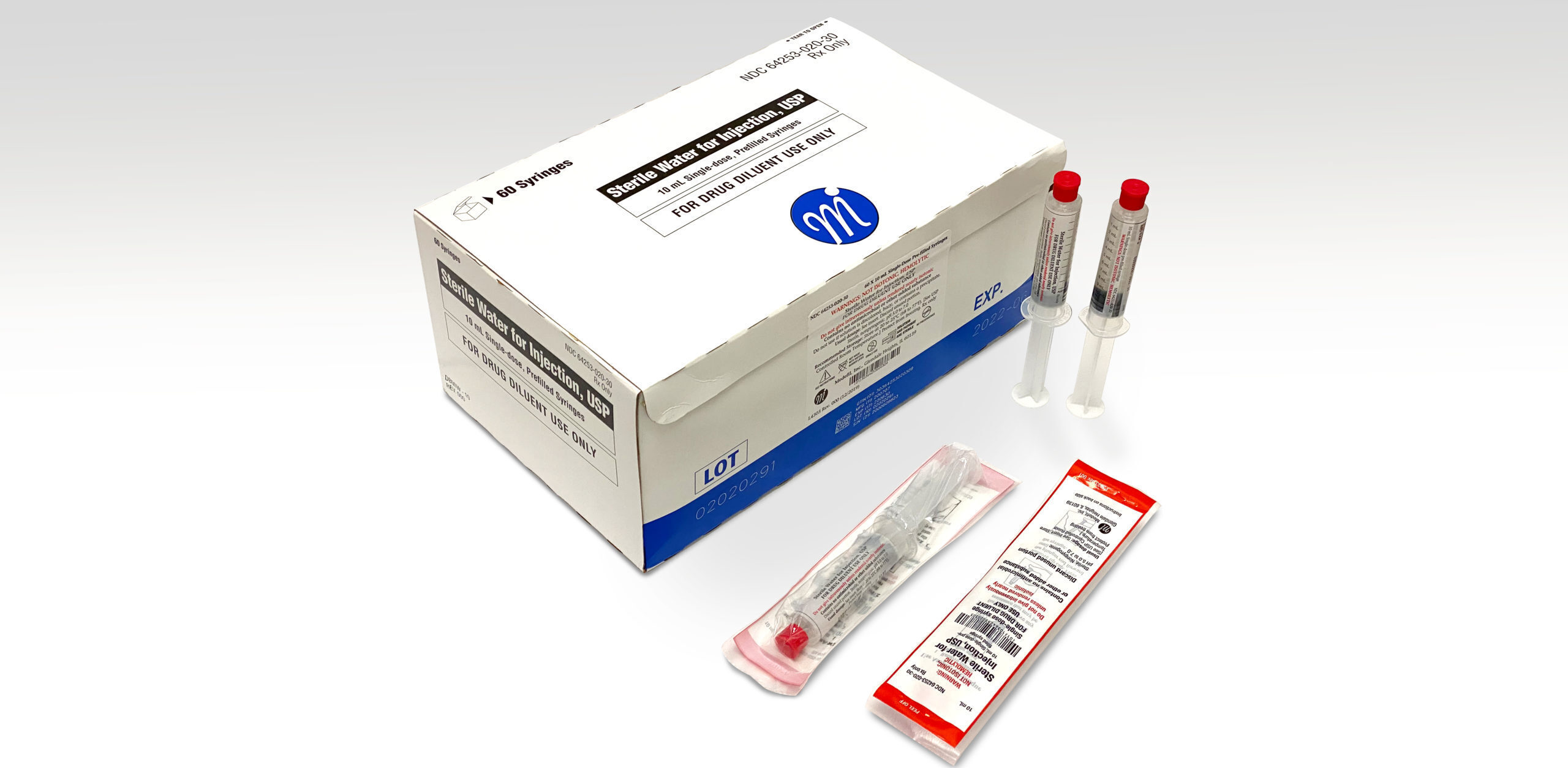
Medefil, Inc. was incorporated in 1998 with the focus on addressing the unmet need for saline and heparin flush injectable products. Medefil’s commitment to providing high quality, cost-effective sterile pharmaceutical products led us to install two state-of-the-art automated syringe manufacturing lines. To-date Medefil has supplied over one billion sterile prefilled syringe products manufactured by these automated syringe lines.
After spending over 15 years establishing manufacturing excellence, Medefil made the decision to begin formulation and development of injectable products. Medefil, now has a state-of-the-art R&D lab with the capability of developing injectable products and is now installing additional manufacturing lines that will give us the ability to supply high-quality, cost-effective injectable products.
Medefil’s History
Our Mission
Always Innovating
We are a specialty pharmaceutical company dedicated to improving patient safety and quality of life by developing and manufacturing high-quality and affordable injectable products that fulfill a critical unmet market need.
Our Vision
Medefil strives to identify critical unmet needs in medication delivery and resolve them.
Our Business Model
Medefil is growing as a fully integrated specialty pharmaceutical company. At Medefil our goal is to develop and manufacture injectable products where quality and supply reliability are crucial, and safety and ease-of use are of utmost importance.
Medefil is committed to building a diversified pipeline that aligns with Medefil’s philosophy for success:
Critical Injectable
Injectable with shortage and supply issues
Complex Injectable
Difficulty to develop and manufacture injectable products
Product Enhancement
Enhancement of injectable product to create value at the point of care