Our Development Capabilities
Medefil has established a state of the art R&D facility to develop injectable products. The laboratory scientific staff possesses a good blend of experience and expertise for developing injectable drugs across different formats which can be scaled up to Medefil’s existing and upcoming manufacturing facility. The laboratory includes capability to develop injectable drugs in different container closure system including plastic and glass vials, plastic and glass syringes, and pre-mixed bags.

- Capabilities to develop different platform dosage forms: solutions, suspensions, pre-mixed bags, and lyophilized products
- Specialized in developing Combination Products including plastic and glass pre-filled syringes
- Specialized compounding tanks including jacketed vessels, single use manufacturing system, filtration and semi-automatic filling under laminar flow hood

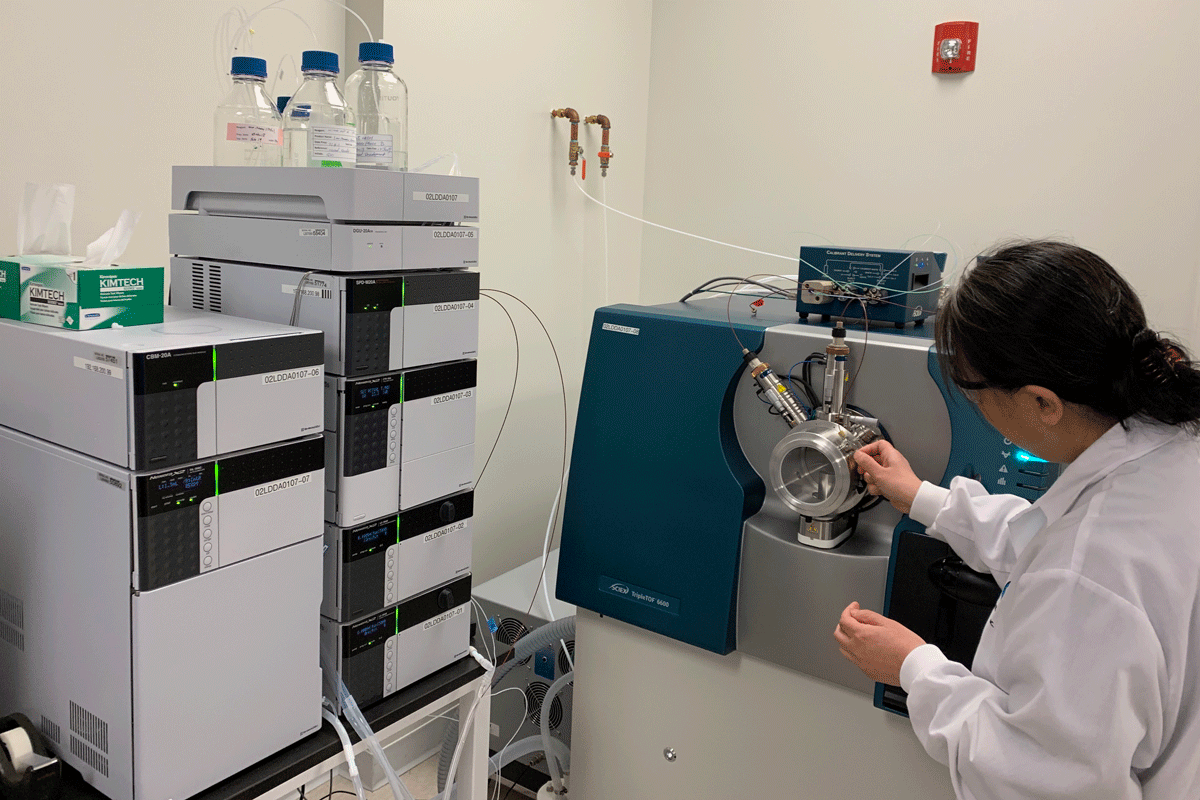
- Chromatographic systems (HPLCs, UPLCs, IC, CAD, GPC, and GCs) and Mass Spectroscopy
- Dissolution capability including USP APP-2 and USP APP-4
- Various wet chemistry and identification techniques: Titrations, KF, ROI, LOD, UV, FTIR, Polarimeter
- Extraction equipment’s including reflux vessels, rotary evaporator.
- Mass Spectroscopy for elemental extractable and leachable impurities by ICPMS
- Mass Spectroscopy for volatile and semi-volatile extractable and leachable impurities by GC-MS
- Mass Spectroscopy for non-volatile extractable and leachable impurities by LC-MS/MS-TOF
